Editor's Note: The original version of this article was published by Rice News on April 18, 2022.
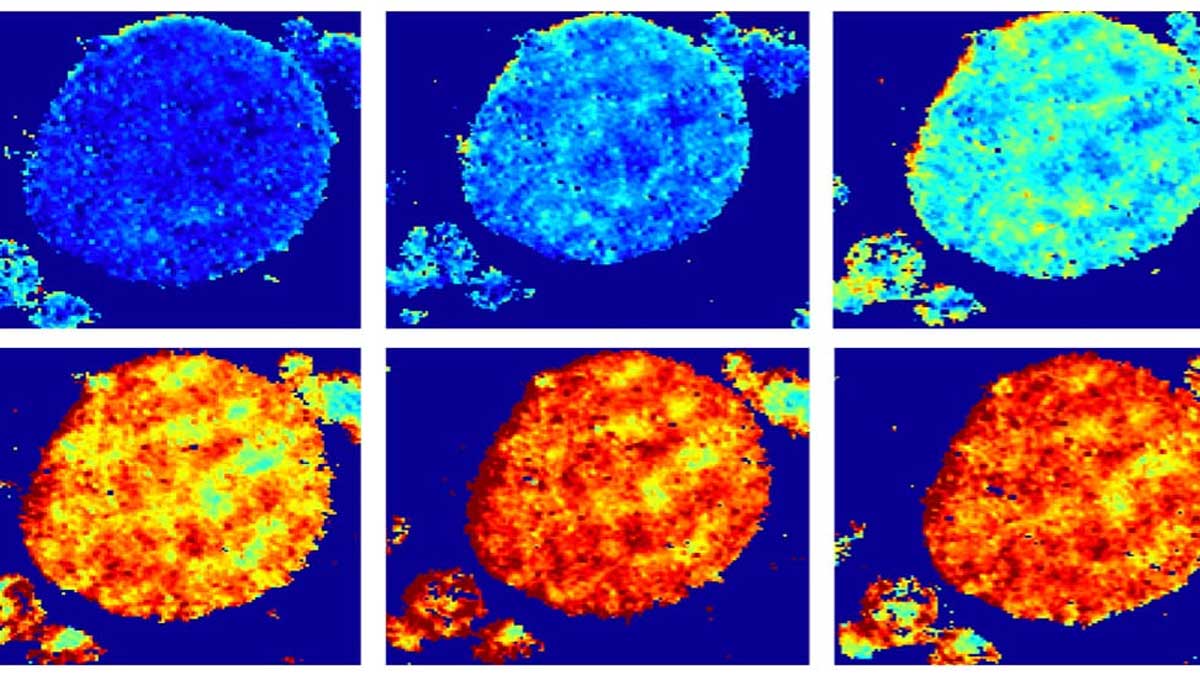
If you could shrink enough for a fantastic voyage across a lithium battery electrode, you would notice the level of charge at every scale is highly uneven.
That’s not good news for the battery’s health. Ming Tang, associate professor of materials science and nanoengineering (MSNE) at Rice, recognized the problem and worked with the U.S. Department of Energy to view in detail how the various particles in an electrode interact with lithium during use.
His research group analyzed nano- and micro-scale interactions within lithium iron phosphate cathodes through modeling and imaging offered by the transmission X-ray microscopy capabilities at Brookhaven National Laboratory and Argonne National Laboratory.
Their paper in the American Chemical Society journal ACS Energy Letters supports theories Tang and his colleagues formed several years ago that foresaw how lithium travels in the dynamic environment inside a typical commercial cathode.
Being able to watch sealed cathodes charge and discharge at Brookhaven offered absolute proof.
“Batteries have a lot of particle aggregates that soak up and give up lithium, and we wanted to know what happens on their surfaces, how uniform the reaction is,” Tang said. “In general, we always want a more uniform reaction so we can charge the battery faster.”
In images taken at Brookhaven’s powerful X-ray synchrotron, the researchers observed that some regions inside the cathode were better at absorption than others. The ability to look at single or aggregated particles in 3D showed that rather than reacting over their entire surfaces, lithium favored particular regions more than others.
“This is very different from conventional wisdom,” Tang said. “The most interesting observation is that these reaction regions are shaped like one-dimensional filaments lying across the surface of these aggregated particles. It was kind of weird, but it matched what we saw in our models.”
The lithium filaments resembled thick nanotubes and measured several hundred nanometers wide and several microns long.
Stress between misaligned crystallites in the particle agglomerates, Tang said, prevents lithium from being uniformly inserted into or extracted from the aggregate surface because that will generate too large an energy penalty. Instead, lithium is forced to flow into or out of the aggregates at “hot spots” that develop the filament shape.
“This is a bad thing,” Tang said. “Because the lithium can’t go into the cathode uniformly, it slows down the intercalation mechanics.”
“What our study offers are potential ways to help make lithium insertion or extraction more uniform on these aggregates or individual particles,” Tang said. "Introducing porosity in the particle agglomerates might sacrifice some energy density, but would allow lithium to go in more uniformly. That could allow you to get more energy at a given charge/discharge rate.”
“If we can somehow align the orientation of these small particles so their maximum expansion is perpendicular to each other, they’ll better accommodate lithium intercalation,” he said, admitting that would pose a challenge for battery manufacturers.
“We don’t have enough experience in synthesis to know how to make that happen,” Tang said. “What we’re providing is bait. Let’s see if somebody bites.”
Rice graduate alumni Fan Wang and Kaiqi Yang are co-lead authors of the paper. Co-authors are Mingyuan Ge, Jiajun Wang, Jun Wang, Xianghi Xiao and Wah-Keat Lee, all of Brookhaven National Laboratory, Upton, New York; and Linsen Li of Shanghai Jiao Tong University.
The Department of Energy, Basic Energy Sciences and the National Science Foundation supported the research.
by Mike Williams, Rice University Public Affairs